An old mouse (front) already displaying some physical limitations. Muscle atrophy can be accelerated due to long periods of inactivity.
(image from Jeff Miller, University of Wisconsin-Madison)
(image from Jeff Miller, University of Wisconsin-Madison)
In a paper published in The Journal of Biological Chemistry this past October, senior author Dr. Chris Adams and his colleagues (including Michael Dyle, Steven Bullard, Jason Dierdorff, Daryl Murry, Daniel Fox, Kale Bongers, Vitor Lira, David Meyerholz, Scott Ebert, and John Talley) went through a compound library of 1,309 compounds to look for small molecule candidates that might have potential in mediating muscle atrophy resulting from fasting or spinal cord injury. Life events such as these are major factors leading to muscle atrophy.
Left to right are CM Adams, JJ Talley, and SM Ebert who are co-authors of the paper explaining how they discovered tomatidine and ursolic acid as small molecules that would mediate age-related skeletal muscle weakness. They are now 3 of the 4 key people in a new company called Emmyon, a "biotechnology company that discovers and develops natural and
pharmaceutical compounds that improve muscle mass, strength, exercise
capacity, and metabolism." (image from Emmyon site)
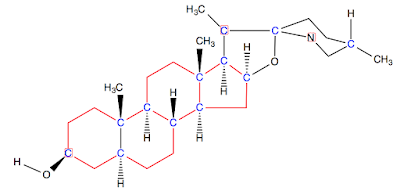
Tomatidine is a naturally occurring compound found in green tomatoes and
green apples. Up to 0.5 g of tomatidine can occur per kilogram of fresh
green tomato. The compound itself is a steroid as well as an alkaloid.
In this colored image of tomatidine, chiral centers are blue and the steroid part is red. The alkaloid part is on the far right and is classified based on the piperidine carbon and nitrogen skeleton.
Zebra tomatoes are naturally green when ripe and are a good source of tomatidine.
Tomatidine is a product of hydrolysis from tomatine, a larger molecule that also includes some sugars. This larger compound is thought to play a protective role in tomato plants against fungi, bacteria, viruses, and insects.
Tomatine has been studied for its potential role against fungi, bacteria, viruses, and insects. Can you see which bond breaks in its hydrolysis to get tomatidine?
(image by
Edgar181 (Own work) [Public domain], via Wikimedia Commons)
Another compound that the Iowa research team studied for its potential to reduce muscle atrophy is ursolic acid, a compound containing 5 hydrocarbon rings and is known as a triterpenoid. As a triterpenoid, this molecule is made from 6 isoprene units.
Ursolic acid, chiral carbons are blue
In a plant, biochemical pathways take 6 isoprene molecules and turn them into a pentacyclic ursolic acid compound.
(image from http://www.mdpi.com/2072-6651/2/10/2428/htm)
The peels of Fuji and Granny Smith apples are common sources of ursolic acid.
(Images
by Taken
by
fir0002 | flagstaffotos.com.auCanon 20D
+ Sigma 150mm f/2.8 - Own work; by Takeaway - Own work. Licensed under CC
BY-SA 4.0)
So what did C. M. Adams and his team discover when adult (6 month) and senior-age (22 month old) mice ingested tomatidine (0.05%) or ursolic acid (0.27%) in their diet? Both had similar effects in that the mice gained skeletal muscle weight by 9-10%, increased the size of type IIb or fast-twitch muscle fibers in the quadriceps, and improved forelimb grip strength by 10-12%.
The researchers in another paper suggest that these observations may may due to the inhibitory effect of tomatidine and ursolic acid on ATF4, a transcription factor known to activate several cell stress responses and may be responsible for muscle atrophy. More research is being done.
*Identification and Small Molecule Inhibition of an Activating Transcription Factor 4 (ATF4)-dependent Pathway to Age-related Skeletal Muscle Weakness and Atrophy; SM Ebert, MC Dyle, SA Bullard, JM Dierdorff, DJ Murry, DK Fox, KS Bongers, VA Lira, DK Meyerholz, JJ Talley, and CM Adams; Journal of Biological Chemistry; vol. 290, no. 42, pp 25497-25511.
*Systems-based Discovery of Tomatidine as a Natural Small Molecule Inhibitor of Skeletal Muscle Atrophy; MC Dyle, SM Ebert, DP Cook, SD Kunkel, DK Fox,
KS Bongers, SA Bullard, JM Dierdorff, and CM Adams; Journal of Biological Chemistry; vol. 289, no. 21, pp 14913-14924.
The researchers in another paper suggest that these observations may may due to the inhibitory effect of tomatidine and ursolic acid on ATF4, a transcription factor known to activate several cell stress responses and may be responsible for muscle atrophy. More research is being done.
In this fake commercial for Nolan's Cheddar, John Nolan (the animatronics wiz of Where the Wild Things Are) created a workout for this rat empowered after eating some of the cheese. There is no known publication on the effect of cheese on muscle strength. The video clip is for inspiration. (image from https://vimeo.com/12106387).
For More Information:
*Identification and Small Molecule Inhibition of an Activating Transcription Factor 4 (ATF4)-dependent Pathway to Age-related Skeletal Muscle Weakness and Atrophy; SM Ebert, MC Dyle, SA Bullard, JM Dierdorff, DJ Murry, DK Fox, KS Bongers, VA Lira, DK Meyerholz, JJ Talley, and CM Adams; Journal of Biological Chemistry; vol. 290, no. 42, pp 25497-25511.
*Systems-based Discovery of Tomatidine as a Natural Small Molecule Inhibitor of Skeletal Muscle Atrophy; MC Dyle, SM Ebert, DP Cook, SD Kunkel, DK Fox,
KS Bongers, SA Bullard, JM Dierdorff, and CM Adams; Journal of Biological Chemistry; vol. 289, no. 21, pp 14913-14924.